Soil ph charts
The Four Things You Need to Know About Soil pH
Don’t be too quick to blame horrendous-sounding afflictions like “verticillium” and “fusarium” or any other diseases for the sickly yellowing of your pin oak’s or geranium’s leaves. The problem may be that your soil’s pH is out of whack. Every plant has its preferred range of soil acidity, and when the pH level is out of that range, a host of ills may follow. A basic understanding of pH will not only help keep your garden healthy but also assist you if things go bad. Here is what you need to know to make smart decisions about managing your soil’s pH.
1. What is pH?
The acidity or alkalinity of a substance is measured in pH units, a scale running from 0 to 14. A pH of 7 is neutral. As numbers decrease from 7, the acidity gets higher. As numbers increase from 7 so does the alkalinity. Soils generally range from an extremely acidic pH of 3 to a very alkaline pH of 10. This range is a result of many factors, including a soil’s parent material and the amount of yearly rainfall an area receives. Most cultivated plants enjoy slightly acidic conditions with a pH of about 6.5. Pin oak, gardenia, blueberry, azalea, and rhododendron are among the plants that demand a very acidic pH of 4.5 to 5.5.
2. What does pH do? Soil pH has indirect yet far-reaching effects on plants. Plant nutrients become available or unavailable according to the soil’s pH level (chart, right). Yellowing between the veins of young leaves indicates an iron deficiency, a condition arising not from a lack of iron in the soil but from insufficient soil acidity to put iron into a form that a plant can absorb. Most plants thrive in slightly acidic soil because that pH affords them good access to all nutrients.
The darker side of soil pH is plant poisoning. Too low a pH level can render the plant nutrient manganese available at toxic levels; geraniums are particularly sensitive to this, showing their discomfort with yellowed, brown-flecked, or dead leaves. A pH level that is too low also liberates aluminum—not a plant nutrient—in amounts that can stunt root growth and interfere with a plant’s uptake of nutrients.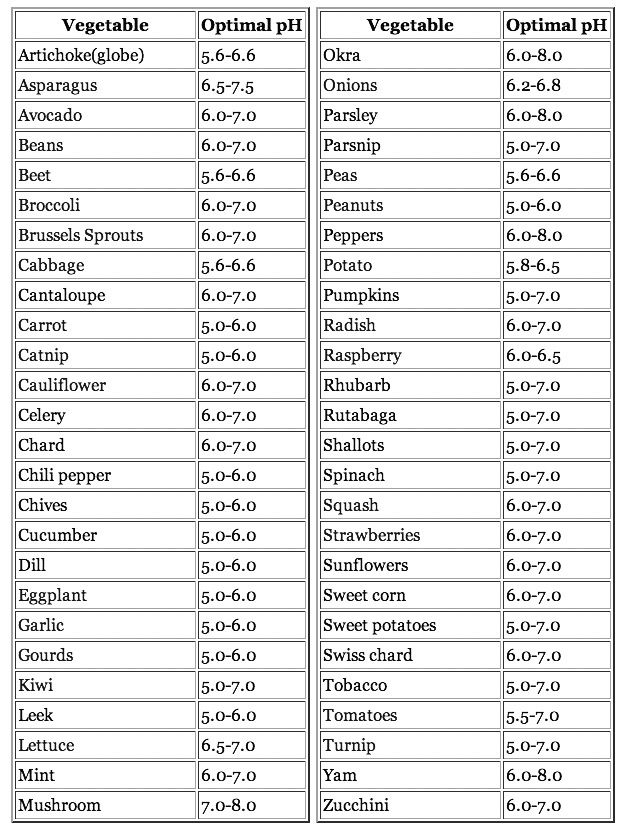 At a high pH level, the plant nutrient molybdenum becomes available in toxic amounts.
At a high pH level, the plant nutrient molybdenum becomes available in toxic amounts.
Soil pH also influences soil-dwelling organisms, whose well-being, in turn, affects soil conditions and plant health. The slightly acidic conditions enjoyed by most plants are also what earthworms like, as do microorganisms that convert nitrogen into forms that plants can use.
3. How do you adjust your pH?
Before attempting to change your soil’s pH, you must know its current level. This will determine how much you need to raise or lower it, if at all. A simple soil test can be done at home or by a soil-testing laboratory. You must also know your soil’s texture, be it clay, sand, or something in between. More material is needed to change the pH level of a clay soil than for a sandy soil because the charged surfaces of clays make them more resistant to pH changes than the uncharged surfaces of sand particles.
Generally, limestone is used to raise a pH level, and sulfur is used to lower it.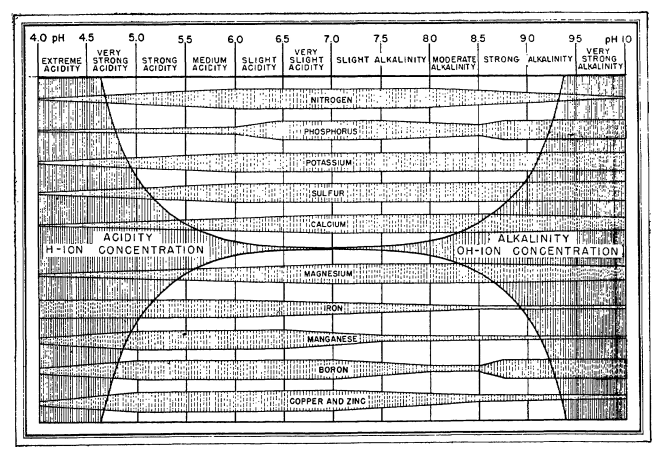 Limestone is relatively pure calcium carbonate, but dolomitic limestone is a mix of calcium carbonate and magnesium. Pound for pound, dolomitic limestone neutralizes more acidity than pure limestone and adds magnesium to the soil, perfect for those who garden in the East or the Pacific Northwest where this nutrient is naturally low.
Limestone is relatively pure calcium carbonate, but dolomitic limestone is a mix of calcium carbonate and magnesium. Pound for pound, dolomitic limestone neutralizes more acidity than pure limestone and adds magnesium to the soil, perfect for those who garden in the East or the Pacific Northwest where this nutrient is naturally low.
Limestone and sulfur are available in powdered or pelletized form, with the latter being easier to spread uniformly and causing less of a health hazard from dust. Avoid using powdered sulfur sold as a fungicide because it is finer and more expensive than needed for acidifying soil. Neither limestone nor sulfur is soluble in water, so mix these materials thoroughly into the top 6 inches of soil when quick action is needed. Otherwise, just lay the material on top of the ground, and let it gradually work its way down.
4. Why should you monitor your pH? Once the pH level is adjusted for the plants you are growing, do not put it out of your mind.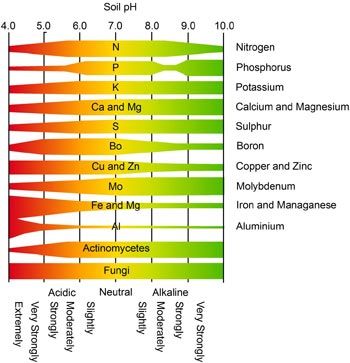 Maintaining the correct pH level for your soil is an ongoing task, especially in the naturally acidic soils of the East and the Northwest, where rainfall leaches out calcium and other alkaline-forming elements. Naturally alkaline soils will keep shifting up the pH scale because of the rock minerals from which they were formed. In some cases, acidifying these soils is unfeasible. Even fertilizers can shift your soil pH over time, with materials such as ammonium sulfate and ammonium nitrate pushing the pH level lower and potassium nitrate or calcium pushing the value higher. Hence, there’s a need for regular additions of limestone or sulfur.
Maintaining the correct pH level for your soil is an ongoing task, especially in the naturally acidic soils of the East and the Northwest, where rainfall leaches out calcium and other alkaline-forming elements. Naturally alkaline soils will keep shifting up the pH scale because of the rock minerals from which they were formed. In some cases, acidifying these soils is unfeasible. Even fertilizers can shift your soil pH over time, with materials such as ammonium sulfate and ammonium nitrate pushing the pH level lower and potassium nitrate or calcium pushing the value higher. Hence, there’s a need for regular additions of limestone or sulfur.
Most Popular
View Comments
Design
How to Create a Butterfly Haven
I will never forget when I became hooked on butterflies. It was 2007, my first year working at Powell Gardens in Kingsville, Missouri. Each spring the garden includes milkweeds (Asclepias…
It was 2007, my first year working at Powell Gardens in Kingsville, Missouri. Each spring the garden includes milkweeds (Asclepias…
Subscribe today and save up to 61%
Subscribe"As a recently identified gardening nut I have tried all the magazines and this one is head and shoulders above the pack."
Soil pH for Vegetables. Find Soil ph Level for Your Favorite Garden Plants.
About Ideal Soil pH for Vegetables
Gardeners strive to grow the very best and most productive garden plants. It begins with identifying the ideal garden soil pH level for vegetables that you plan to grow. Ideal soil pH levels vary by plant. Essentially, it provides an optimum soil condition to help your plants reach their optimum growth potential.
Start your gardening season right, with a pH soil test.
Chart of Ideal Soil pH for Vegetables
| Vegetables: | Ideal Soil pH |
| Artichoke | 6.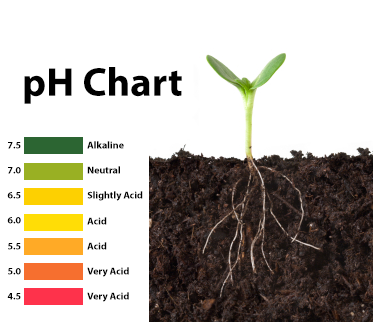 5 – 7.5 5 – 7.5 |
| Arugula (Roquette) | 6.0 – 6.8 |
| Asparagus | 6.0 – 8.0 |
| Beans | 6.0 – 7.5 |
| Beet Root | 6.0 – 7.5 |
| Broccoli | 6.0 – 7.0 |
| Brussels Sprouts | 6.0 – 7.5 |
| Cabbage | 6.0 – 7.5 |
| Carrot | 5.5 – 7.0 |
| Cauliflower | 5.5 – 7.5 |
| Celery | 6.0 – 7.0 |
| Chickpeas | 5.3 – 7.0 |
| Chicory | 5.0 – 6.5 |
| Chinese Cabbage | 6.0 – 7.5 |
| Corn | 5.5 – 7.0 |
| Cress | 6.0 – 7.0 |
| Cucumber | 5.5 – 7.5 |
| Eggplant | 6.0 – 7.0 |
| Garlic | 5.5 – 7.5 |
| Horseradish | 6.0 – 7.0 |
| Kale | 6.0 – 7.5 |
| Kohlrabi | 6.0 – 7.5 |
| Leek | 6.0 – 8.0 |
| Lentil | 5. 5 – 7.0 5 – 7.0 |
| Lettuce | 6.0 – 7.0 |
| Mushroom | 6.5 – 7.5 |
| Mustard | 6.0 – 7.5 |
| Onion | 6.0 – 7.0 |
| Parsley, Hamburg Rooted | 6.0 – 7.0 |
| Parsnip | 5.5 – 7.5 |
| Pea | 6.0 – 7.5 |
| Peanut | 5.0 – 6.5 |
| Pepper | 5.5 – 7.0 |
| Potato | 4.5 – 6.0 |
| Potato- Sweet | 5.5 – 6.0 |
| Pumpkin | 5.5 – 7.5 |
| Radish | 6.0 – 7.0 |
| Rhubarb | 5.5 – 7.0 |
| Rice | 5.5 – 6.5 |
| Shallot | 5.5 – 7.0 |
| Soybean | 5.5 – 6.5 |
| Spinach | 6.0 – 7.5 |
| Tomato | 5.5 – 7.5 |
| Turnip | 5.5 – 7.0 |
| Water Cress | 5.0 – 8.0 |
| Watermelon | 5.5 – 6.5 |
Note: The most ideal level, is the mid-point of a given range.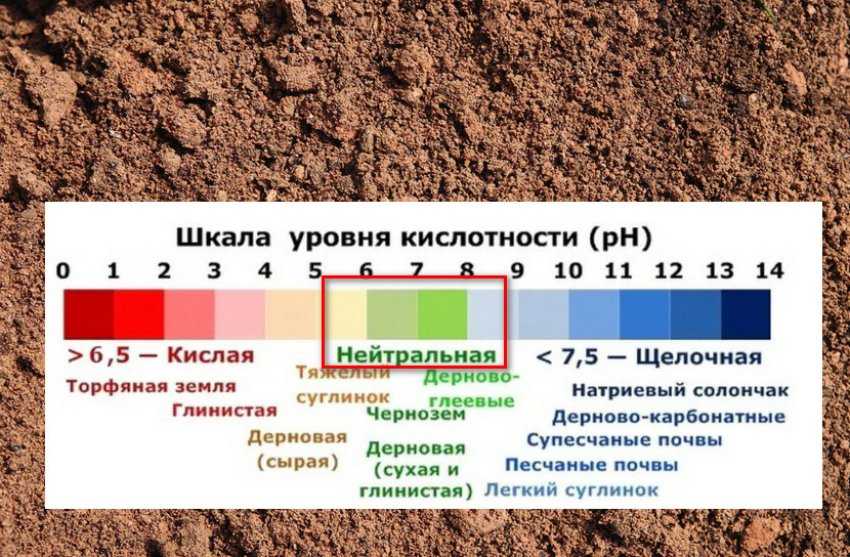
Simple, easy-to-use soil testers, help you to quickly determine if your soil pH needs an adjustment. And, they are inexpensive. About Soil Testers.
Find Ideal Soil pH Levels for your Favorite Plants
Related Articles
Subscribe To Our Newsletter
Please support our site. Shop for:
Composters
Seed Trays
Clothing – Fashions
Kitchenware
Garden Seeds & Supplies
Soil Testers
Electronic Best Sellers
Live Plants
Cell Phones
Groceries
construction, repair, real estate, landscaping
To eliminate the daily dynamics of these values, we carried out measurements in the period from 10:00 to 16:00, when changes in the composition of the liquid phase of soils are minimal.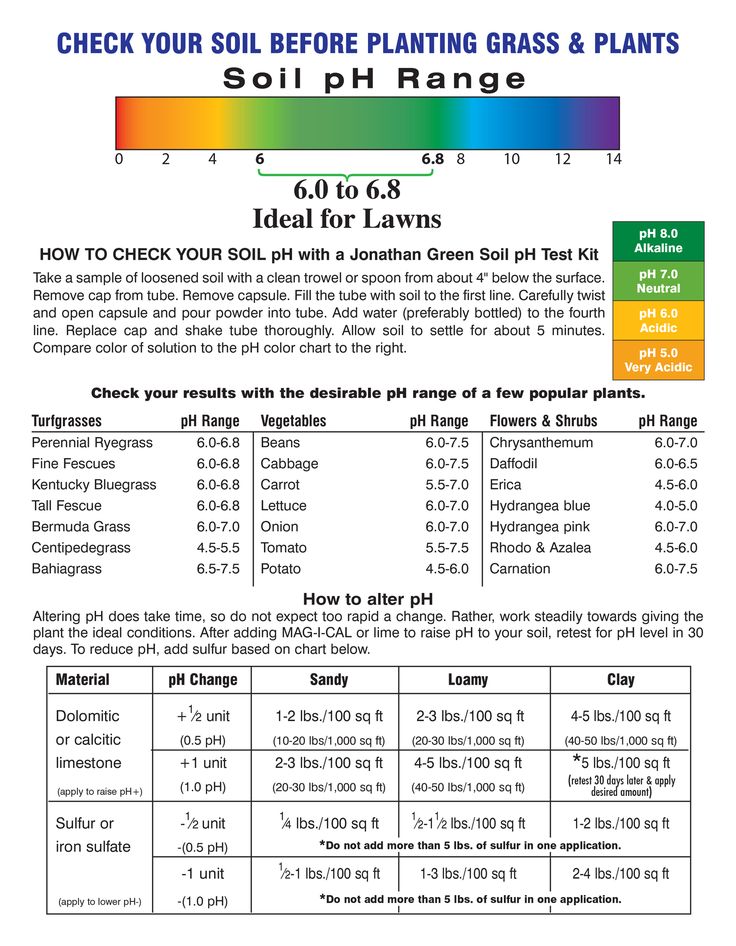 Regarding the influence of soil moisture, it was previously found that it is not so great for the studied parameters. After a heavy downpour, the value of the redox potential of the virgin chernozem ordinary in a day completely reached the initial state (see Fig. 16). The pH value of the liquid phase of the virgin chernozem ordinary returns to its original value 5-6 hours after precipitation; in gray forest soil under conditions of undisturbed herbage, this process takes 1–2 days. Thus, in order to obtain the physicochemical parameters characteristic of this type of soil formation, in situ measurements should be carried out 1–2 days after precipitation. The last condition has always been observed by us.
Regarding the influence of soil moisture, it was previously found that it is not so great for the studied parameters. After a heavy downpour, the value of the redox potential of the virgin chernozem ordinary in a day completely reached the initial state (see Fig. 16). The pH value of the liquid phase of the virgin chernozem ordinary returns to its original value 5-6 hours after precipitation; in gray forest soil under conditions of undisturbed herbage, this process takes 1–2 days. Thus, in order to obtain the physicochemical parameters characteristic of this type of soil formation, in situ measurements should be carried out 1–2 days after precipitation. The last condition has always been observed by us.
The presence of Eh and pH values characteristic of soil-forming processes in various subtypes of chernozems is also shown by the histogram of their distribution (Fig. 43). The redox potential reveals two clear peaks: about 550 mV, associated with ordinary chernozems. and about 610 mV - with typical chernozems.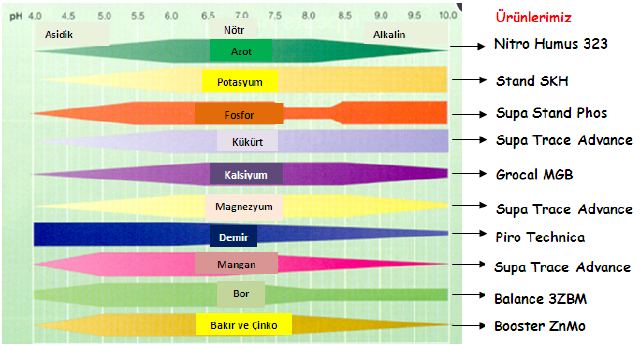 For pH, these peaks are also traced: 5.5 for typical chernozem and 7.2 for ordinary chernozem (Fig. 43a). Leached chernozems were not reflected in the histogram due to the small sample size. An analysis of the histogram constructed on the basis of the primary data of individual electrodes (see Fig. 43b) additionally shows the presence of a maximum on the pH scale near the value of 6.4, due to residual solonetsity (sections 11, 110.481), and an Eh peak in the region of 490 mV associated with chernozem solonetzes.
For pH, these peaks are also traced: 5.5 for typical chernozem and 7.2 for ordinary chernozem (Fig. 43a). Leached chernozems were not reflected in the histogram due to the small sample size. An analysis of the histogram constructed on the basis of the primary data of individual electrodes (see Fig. 43b) additionally shows the presence of a maximum on the pH scale near the value of 6.4, due to residual solonetsity (sections 11, 110.481), and an Eh peak in the region of 490 mV associated with chernozem solonetzes.
Analysis of the Eh-pH diagram of the studied soils (see Fig. 42) shows that the fields of typical and ordinary chernozems are located approximately along the same line corresponding to the equation:
Eh = 871 - 44 pH.
This. in all likelihood, is evidence of the relatedness of the redox reactions that determine their formation. If we proceed from the position that ordinary chernozems differ from typical ones only by a somewhat weakened accumulation of humus, then this equation may characterize the total reaction of humus accumulation.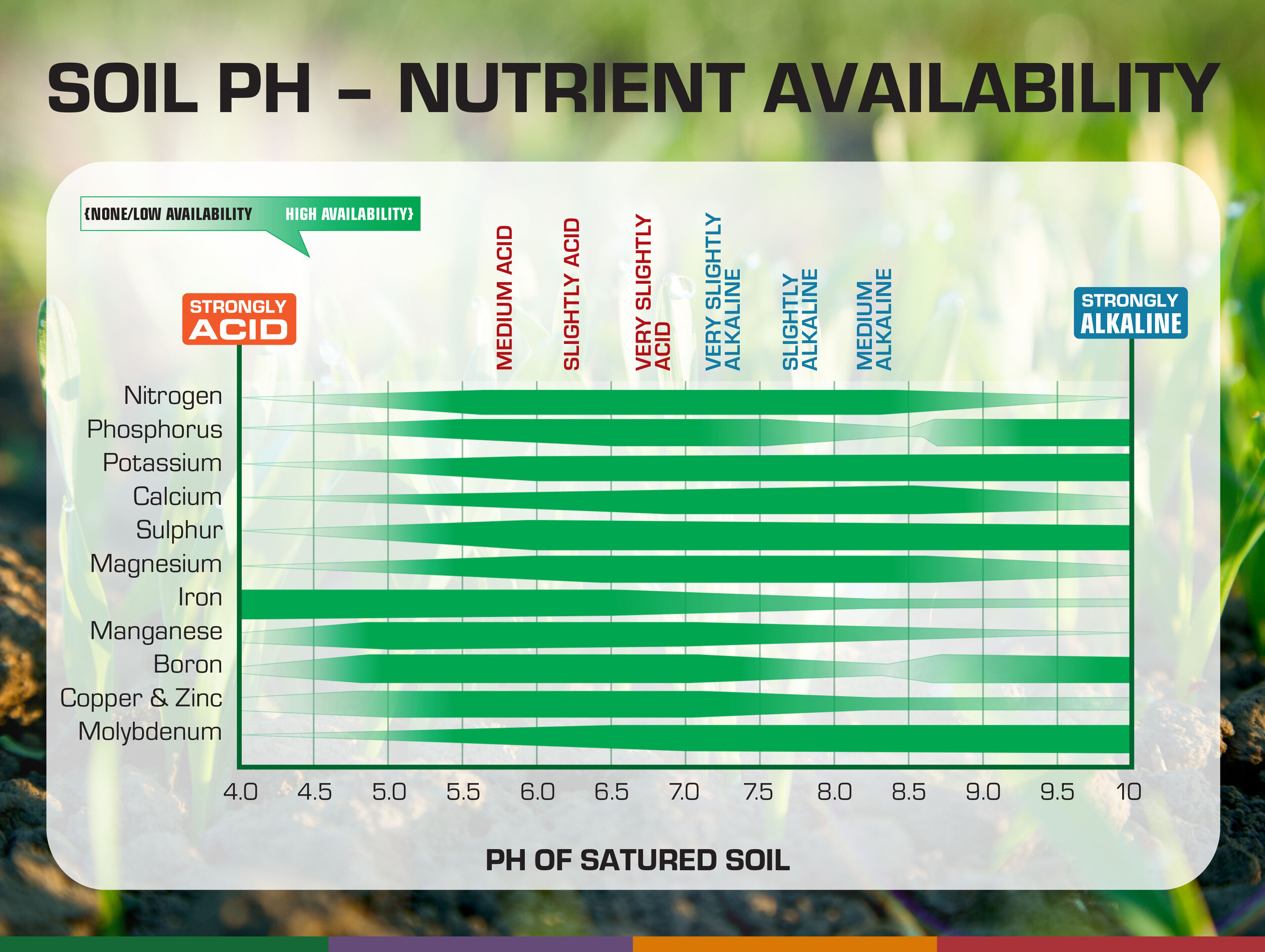 However, the authors of the work, who obtained regressions very close to this equation during artificial acidification and alkalization of various soils (including the one closest to ours for washed quartz sand), consider this to be evidence of the predominant influence of the water potential in the redox environment of normally aerated soils as the most capacious redox system.
However, the authors of the work, who obtained regressions very close to this equation during artificial acidification and alkalization of various soils (including the one closest to ours for washed quartz sand), consider this to be evidence of the predominant influence of the water potential in the redox environment of normally aerated soils as the most capacious redox system.
The area of leached chernozems turned out to be elongated in a different direction. And although the statistics are negligible here, it is likely that there is another mechanism that determines their specificity, in which a change in the pH value is not associated with a significant change in Eh. Apparently, this is the process of washing out carbonates from the humus horizon, a process that does not have a redox nature.
Having considered the data obtained, it can be argued that the values of the redox potential and pH of the liquid phase of soils, measured in situ, have characteristic ranges of values for specific types of soil formation and can be used to identify various subtypes of chernozems.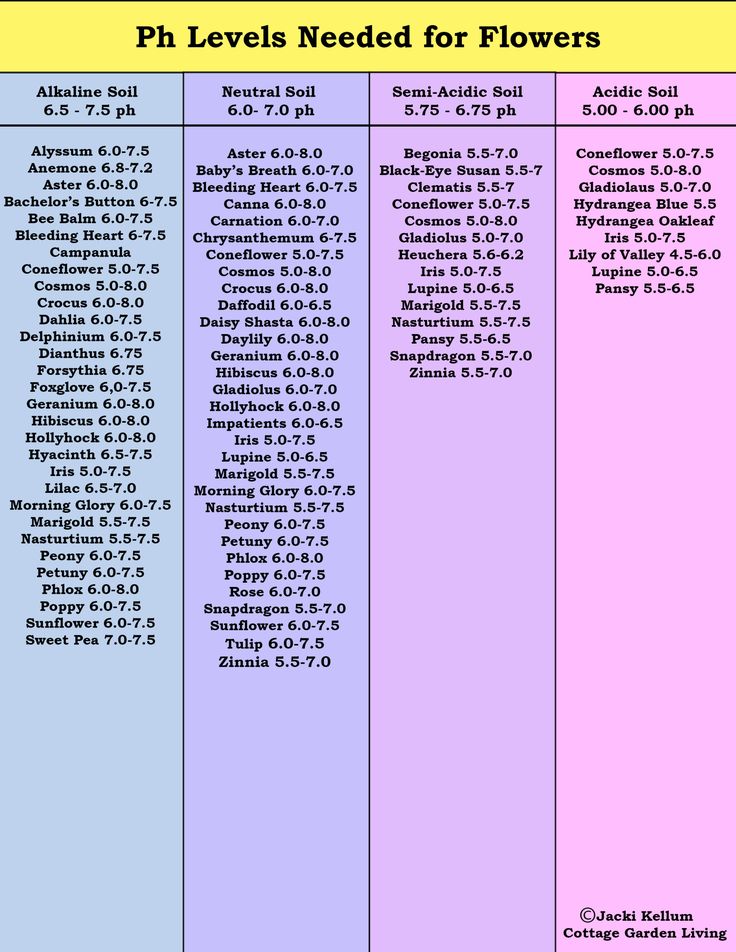
Which PH meter is suitable for determining the acidity of the soil
The “quality of life” of plants, and the microorganisms necessary for their vital activity, largely depends on the ph of the earth. Trees, shrubs, fruit and berry crops receive nutrients from water dissolved in the soil, one of the indicators of which is acidity.
When harvesting hay for livestock, the ph level of the soil during the growing season largely determines the gross harvest of forage grasses (alfalfa, clover, timothy) per hectare and their saturation with vitamins and valuable proteins.
Trace elements are absorbed by plants differently depending on soil acidity. If the pH is not regularly controlled and, for example, the soil is acidic, the delivery of nutrients to plants (roots, stems, leaves, fruits) is difficult.
Example 1. As can be seen from the diagram, the availability of phosphorus decreases when soil pH<6 cellar;

If you know how to measure the ph of the soil, the acidity will be under constant control, which in turn will allow you to take the necessary agronomic measures in a timely manner, in particular, the introduction of mineral and organic additives into the ground, a break of a year or more in the sowing of individual plots (so that the soil " rested").
Litmus testers
The term “hydrogen indicator” (reflecting the ratio of charged positive and negative ions in a solution) appeared in the vocabulary at the beginning of the 20th century, long before the digital ph meter was invented, but the need for measurements already existed and, in principle, the problem was solved. Let the pH indicator could not be determined so accurately, but rather cheaply, without the use of complex, long and expensive laboratory analysis. It was enough to know the elementary foundations of chemistry at the level of the school curriculum.
This is the so-called litmus indicator or test strips
A number of substances (phenolphthalein, litmus) change color depending on the acidity level of the test solution. But you need to have gradations, i.e. "bind" color to numeric values. Let not with an accuracy of PH units (and this is an exponent according to the logarithmic acidity scale), but at least in the range to operate with concepts: acidic soil, neutral, alkaline .
But you need to have gradations, i.e. "bind" color to numeric values. Let not with an accuracy of PH units (and this is an exponent according to the logarithmic acidity scale), but at least in the range to operate with concepts: acidic soil, neutral, alkaline .
But this solution has a number of limitations and the litmus indicator can be recommended rather as an exception:
- This method requires the preparation of a solution, that is, it is necessary to dissolve the selected soil sample. Accordingly, you need at least dishes and, in addition, you must strictly follow the methodology so that the correct concentration is chosen.
- In addition, the water itself, which is used for dilution, also has an initial acidity, which can distort the result.
- The method is not sufficiently accurate and unambiguous - it is necessary that the chemical ph meter is not expired. It also matters the features of human color vision - a subjective factor.

On the other hand, the simplicity and low cost of measuring soil acidity captivate. In fact, you don’t even have to buy ready-made strips - psh indicators. There is a lot of information on the Internet about this, including advice from chemists - gardeners - do-it-yourselfers with reviews and detailed step-by-step recommendations.
Indicator Plants
The preferred habitat for some plants can be neutral, alkaline or acid based soil. These are the so-called plants indicators of soil acidity , the original natural pH meters.
The main advantage is that you do not need to count anything, cook, buy chemicals, spend money or have specialized knowledge. The whole picture is in front of my eyes. Forgive me, take it and see which plants grow, which indicators of soil acidity, how they feel in one or another part of the garden, which means in the same place it makes sense to plant trees, shrubs, vegetables and fruits similar in relation to the same PH indicator .![]()










